Molecular Devices
- Threshold System
Rapid quantitation of biopharmaceutical products or contaminates from bioreactor/fermenters,
downstream processing or final product quality control
The production of biopharmaceuticals, such as monoclonal antibodies, recombinant proteins, vaccines, and nucleic acids
requires special techniques and quality control methods. Electrophoresis, chromatography, and immunochemical methods
are commonly used together to monitor and optimize the biopharmaceutical manufacturing process. Threshold®
Immunoassay System is a dedicated platform for the rapid quantitation of biopharmaceutical products or contaminants from
bioreactors/fermenters, downstream processing and final product quality control. The system is ideal for the quantitation
of proteins, peptides and non-sequence specific total DNA Threshold System
⊙ Pre-configured kits to analyze peptides, proteins and total DNA using a single system
⊙ All reagents provided to label your antibodies or commercially available antibodies quickly and easily
⊙ Assay dynamic range of 2 logs or greater enables the use of the same method from purification to product QC
⊙ High assay sensitivity without the use of radioactive reagents that require expensive disposal
⊙ Assay precision typically <10% for reproducible quantitation of contaminant levels in final product
⊙ Hybridization of the analyte occurs in the liquid phase for faster binding kinetics
⊙ Antigen-antibody complex is captured and concentrated on a membrane to achieve sensitivity equal to or better than
radioimmunoassay, and at least 10 times better than ELISAs
⊙ Rapid sample analysis streamlines media selection and optimization of purification conditions
⊙ Strepavidin's strong affinity for biotin captures and concentrates analyte for high assay selectivity
⊙ The speed and sensitivity of the Threshold System enables the monitoring of protein production during the early stages
of fermentation
⊙ Immunoassay detects protein degradation and conformational changes of protein products
⊙ Accurate quantitation to identify the optimal harvest time to maximize product yield from fermentations
⊙ Validated assays using quality controlled reagents and software
⊙ Software requires individual passwords and user IDs to access, managed access to major features,
audit trail, and signed electronic statements complies with FDA 21 CFR Part 11 requirements
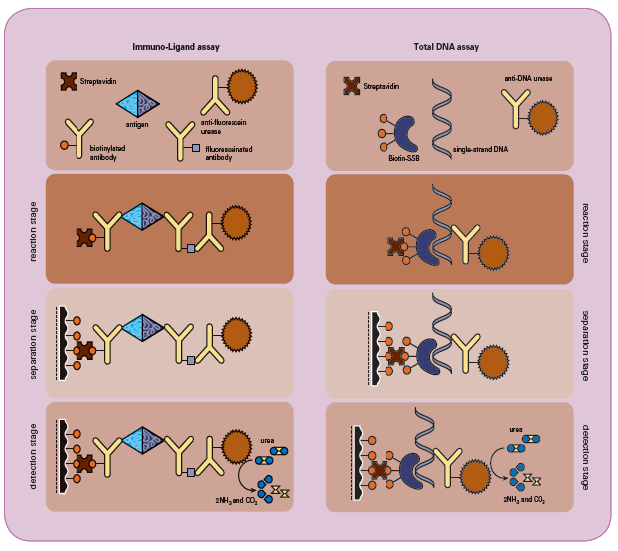
Immuno-Ligand Assay (ILA) measures a broad range of analytes such as, drugs, proteins, and micro-organisms for biopharmaceutical
development and production. Samples can range from fermentation supernatants, samples from a purification process and other
biochemical preparations, to serum or other bodily fluids.
The Total DNA Assay quantitatively measures picograms of single-stranded DNA. Unlike hybridization techniques which require
DNA probes or primers with a specific nucleic acid sequence, Total DNA assay measures DNA with broad sequence specificity.